Laboratory for the Study of Infectious Diseases (LSID) @HWI
Goals
The overarching aim of infectious disease pathogen research at Hauptman-Woodward Institute (HWI) is to investigate basic biological processes of infection and virulence, and to leverage this fundamental knowledge to develop therapeutics and diagnostics. Infectious microorganisms have evolved complex interactions with their host necessary for critical steps of a microorganism's life cycle and for the progression of the infection. Our research currently emphasizes the detection of common themes for virus-host interactions. Future virus-based biological threats are likely to be unanticipated and ill-defined, similar to the unexpected SARS outbreak of 2002-2003. Our research will identify new paradigms for understanding the emergence of viruses via animal-to-human host species jumps, and for developing broad-spectrum therapeutic intervention. Virus-host PPI detection, verification and crystallization technologies will be further developed, and these technologies will be widely applicable to other virus-host systems.
Background
Many infectious diseases, including severe pandemics, have (re-)emerged when an animal virus acquired the ability to infect humans or became significantly more virulent. A survey found 87 new human pathogen species have been reported between 1980-2007 (~3 per year), of which approximately 80% of these are zoonotic (Woolhouse and Gaunt (2007), Crit Rev Microbiol 33, 231-42). Furthermore, 58 (66.6%) of these novel human pathogens are viruses, and 45 (52%) are single-stranded RNA viruses. Thus, viruses in general and specifically single-stranded RNA viruses are greatly over-represented within the pool of emerging human pathogens. Novel future human pathogens will be most likely a) RNA viruses and b) arise from a non-human animal reservoir. Ten to forty new species of human viruses have been predicted to emerge by 2020 based on current rates (Woolhouse et al. (2008), Proc Biol Sci 275: 2111-5). It is highly probable that evolving microorganism threats will include viruses newly emerging from an animal reservoir, and their emergence must be included in the preparation for the future detection and treatment of infectious diseases.
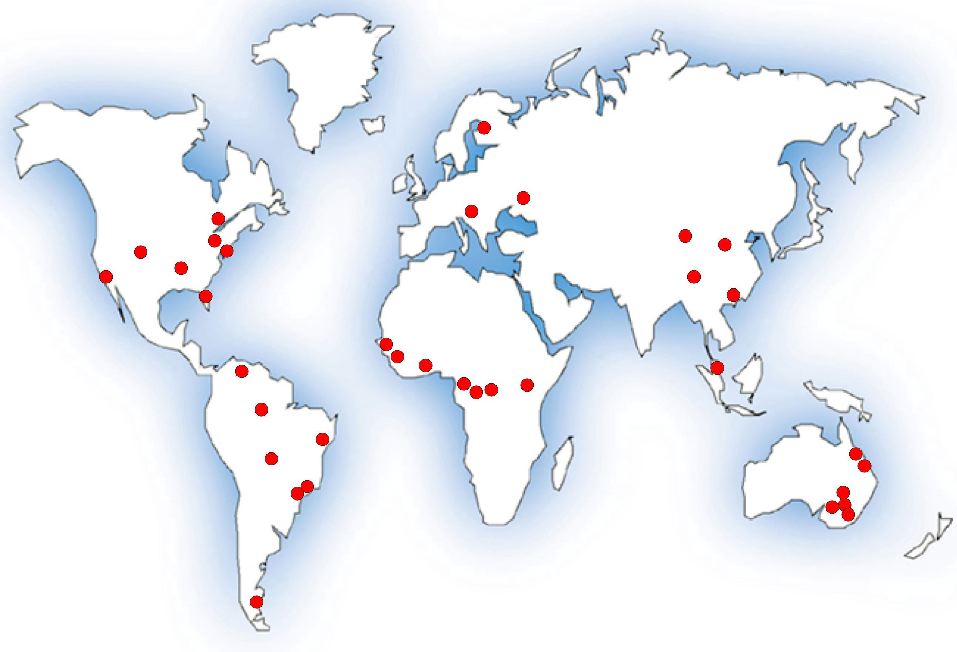 The
emergence of novel human viruses is a global problem. Since 1980, every
continent except Antarctica has experienced multiple distinct events of
emerging human viruses. However, there are specific geographical
hotspots for the general emergence of new human pathogens, and for
particular virus types. For example, new influenza strains tend to
originate in SE Asia. This map presents examples of the origins of the
first reported human cases of novel virus species emerging since 1980.
The map excludes re-emerging viruses. The map is based upon data from
Woolhouse and Gaunt (2007).
The
emergence of novel human viruses is a global problem. Since 1980, every
continent except Antarctica has experienced multiple distinct events of
emerging human viruses. However, there are specific geographical
hotspots for the general emergence of new human pathogens, and for
particular virus types. For example, new influenza strains tend to
originate in SE Asia. This map presents examples of the origins of the
first reported human cases of novel virus species emerging since 1980.
The map excludes re-emerging viruses. The map is based upon data from
Woolhouse and Gaunt (2007).
Research at HWI integrates proteomics, bioinformatics and structural biology techniques to achieve its objectives. A current focus is upon virus-host protein-protein and protein-nucleic acid interactions. The investigation of the molecular interactions between viral macromolecules and host factors is an important yet under-explored frontier in virology, and these complexes are the determinants of species specificity, infectivity and mortality in viral infection. Proteomic methods are used to isolate and identify specific virus-host macromolecular complexes. Bioinformatics analysis of newly identified and previously known virus-host macromolecular complexes will map viral interactions onto human cellular pathways. Structural information is a key component in understanding the effect of viral mutations on the formation of these complexes. As such, structures of these complexes will be determined using highly automated X-ray crystallography and small angle X-ray scattering to reveal structural and sequence determinants critical for the formation the virus-host macromolecular complexes.
Participating Laboratories
 |  |
| Dr. L. Wayne Schultz | Dr. Timothy Umland |
On-Going Research
SARS-CoV NSP9
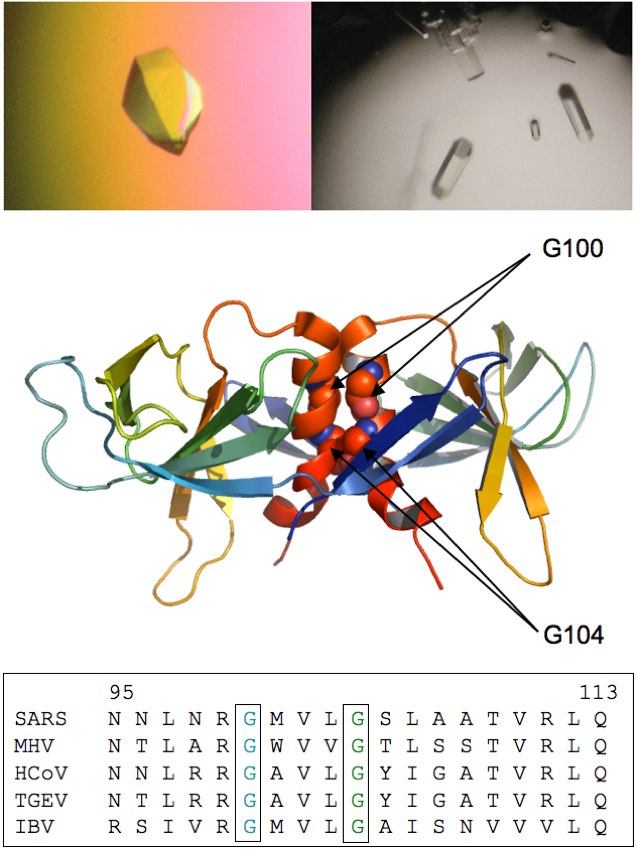 SARS-CoV non-structural protein 9 (nsp9) is an RNA-binding protein which
co-localizes with the viral replication complex. We are interested in
evaluating the biological relevance of several dimerization motifs
observed for SARS-CoV nsp9 within different crystal structures. Nsp9
has been shown to form two different homodimers possessing
oligonucleotide binding capability: a dimer with a helix-helix
interface and one with a sheet-sheet interface. We have investigated
the role of the GXXXG motif that is a critical element in the
formation of the parallel helix-helix dimer interface. This motif is
highly conserved throughout the coronavirus family as demonstrated in
the sequence alignment. Through x-ray crystallography, biophysical
characterization (light scattering, gel filtration, fluorimetry), and
studying the effect mutations to this dimerization motif through a
reverse genetics system (in conjunction with the Baric Lab; UNC), we
have shown that the helix-helix dimer is necessary for efficient virus
replication and that G100 and G104 are critical for this function.
Further work is proceeding in evaluating the residues that are
important for RNA binding by nsp9.
SARS-CoV non-structural protein 9 (nsp9) is an RNA-binding protein which
co-localizes with the viral replication complex. We are interested in
evaluating the biological relevance of several dimerization motifs
observed for SARS-CoV nsp9 within different crystal structures. Nsp9
has been shown to form two different homodimers possessing
oligonucleotide binding capability: a dimer with a helix-helix
interface and one with a sheet-sheet interface. We have investigated
the role of the GXXXG motif that is a critical element in the
formation of the parallel helix-helix dimer interface. This motif is
highly conserved throughout the coronavirus family as demonstrated in
the sequence alignment. Through x-ray crystallography, biophysical
characterization (light scattering, gel filtration, fluorimetry), and
studying the effect mutations to this dimerization motif through a
reverse genetics system (in conjunction with the Baric Lab; UNC), we
have shown that the helix-helix dimer is necessary for efficient virus
replication and that G100 and G104 are critical for this function.
Further work is proceeding in evaluating the residues that are
important for RNA binding by nsp9.
SARS-CoV Repression of the Interferon Response
 The ability of SARS-CoV to successfully replicate within infected cells
depends largely on its ability to evade host defenses. Immune signaling
pathways alerting cells to infection and their need to respond
accordingly are shut down in SARS-infected cells by several mechanisms.
SARS produces a variety of accessory proteins which give the virus a
competitive advantage during infection, often times through modulating
the immune response. One such pathway identified by our
collaborators is through the karyopharin/importin nuclear import
cascade (Frieman
et al. Journal of Virology 81: 9812-9824). The SARS-CoV accessory protein Orf6 binds to the cellular nuclear import protein karyopharin
alpha 2 (KPNA2). The binding of KPNA2 by Orf6 is postulated to
shut down the interferon response in infected cells by sequestering
KPNA2 from the normal nuclear import process. We have produced crystals
of human KPNA2 which are of diffraction quality and are attempting to
generate crystals containing Orf6 and Orf6 derived peptides. KPNA2
is a common host partner in virus-host interactions, and we are
currently studying those interactions.
The ability of SARS-CoV to successfully replicate within infected cells
depends largely on its ability to evade host defenses. Immune signaling
pathways alerting cells to infection and their need to respond
accordingly are shut down in SARS-infected cells by several mechanisms.
SARS produces a variety of accessory proteins which give the virus a
competitive advantage during infection, often times through modulating
the immune response. One such pathway identified by our
collaborators is through the karyopharin/importin nuclear import
cascade (Frieman
et al. Journal of Virology 81: 9812-9824). The SARS-CoV accessory protein Orf6 binds to the cellular nuclear import protein karyopharin
alpha 2 (KPNA2). The binding of KPNA2 by Orf6 is postulated to
shut down the interferon response in infected cells by sequestering
KPNA2 from the normal nuclear import process. We have produced crystals
of human KPNA2 which are of diffraction quality and are attempting to
generate crystals containing Orf6 and Orf6 derived peptides. KPNA2
is a common host partner in virus-host interactions, and we are
currently studying those interactions.
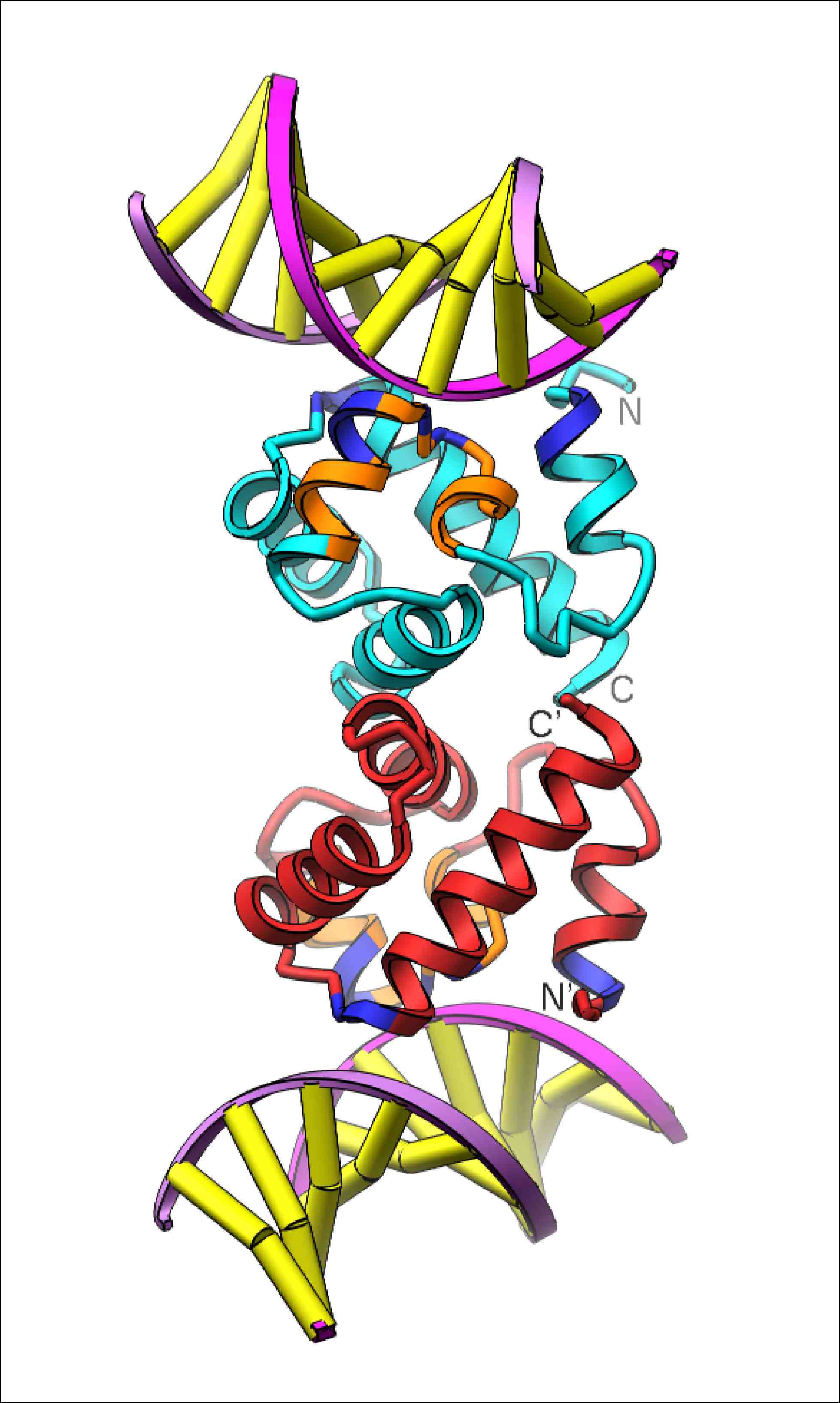
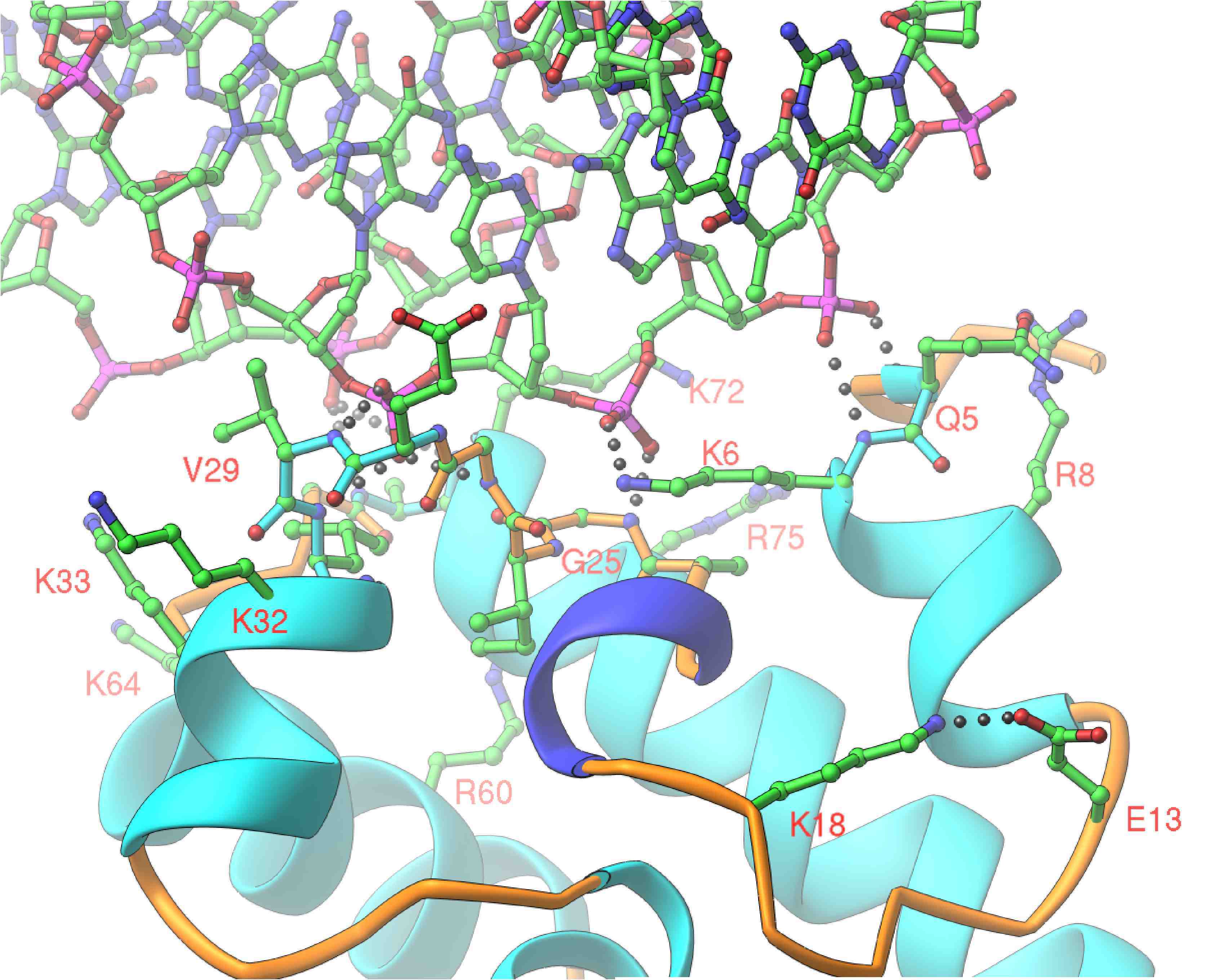
Barrier-to-Autointegration Factor (BAF)
The human protein Barrier-to-Autointegration Factor (BAF) is an essential protein which is capable of bridging dsDNA as well as forming protein-protein interactions with other nuclear proteins. As such, BAF serves to glue together DNA-protein complexes. BAF's name arises from its role in as a host factor in HIV-1 genome integration into the human host's genome. BAF greatly reduces the probability that the reverse-transcribed HIV-1 genome will use itself as the target DNA during intergration (autointegration), leading to significantly higher probability of productive integration into the host's genome. BAF has been recently found to be an important inhibitor of poxvirus replication unless inactivated by the poxvirus B1 kinase (REF), implicating BAF as a common host factor for involved in the replication of viruses which include a dsDNA stage.
 |  |
Hauptman-Woodward Medical Research Institute
With more than 50 years of exceptional scientific research, the Hauptman-Woodward Institute (HWI) is an internationally-renowned independent, non-profit facility specializing in life-altering biomedical research. It is headed by Nobel Laureate Herbert Hauptman. HWI’s 75 team members are committed to improving human health through the study of the causes of diseases, as well as potential therapies, at their fundamental molecular level. HWI is located in the heart of the Buffalo Niagara Medical Campus (BNMC) in downtown Buffalo, New York, in a new state-of-the-art research facility. Our BNMC neighbors include Roswell Park Cancer Institute and the University at Buffalo. In addition to HWI's role as an independent research institute, it houses the University at Buffalo's Department of Structural Biology.